A 0.465 g sample of an unknown compound occupies 245 mL at 298 K and 1.24 bar. What is the molar mass of the unknown compound?
A) 26.3 g 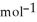
B) 33.9 g 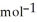
C) 12.2 g 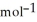
D) 37.9 g 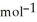
E) 81.8 g 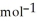
Correct Answer:
Verified
Q21: Define hypoxia.
A) oxygen starvation
B) increased oxygen concentration
Q50: A gas mixture consists of N2,O2,and Ne,where
Q72: A 0.334 g sample of an unknown
Q73: What volume will 0.780 moles of He
Q74: A mixture of 10.0 g of Ne
Q76: A mixture of He, Ne, and Ar
Q77: A mixture of N2, O2, and Ar
Q78: A syringe contains 589 mL of CO
Q79: The following reaction is used to generate
Q80: A mixture of 0.220 moles CO, 0.350
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents