Which of the gases in the graph below has the smallest molar mass? 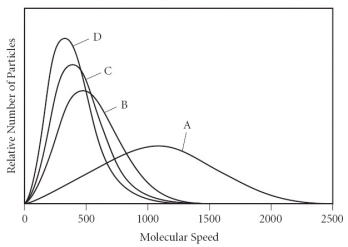
A) A
B) B
C) C
D) D
E) There is not enough information to determine the answer.
Correct Answer:
Verified
Q44: Calculate the ratio of effusion rates of
Q80: Which of the following compounds will behave
Q112: What is the temperature if krypton has
Q113: Calculate the molecular weight of a gas
Q115: Which of the following statements is TRUE?
A)
Q118: Which of the following gases has the
Q119: At what temperature does argon have a
Q120: The ratio of the rate of effusion
Q121: Which of the following would have a
Q122: What is the volume of 30.0 g
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents