Three identical flasks contain three different gases at standard temperature and pressure. Flask A contains 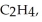 flask B contains O3, and flask C contains F2. Which flask contains the largest number of molecules?
flask B contains O3, and flask C contains F2. Which flask contains the largest number of molecules?
A) flask A
B) flask B
C) flask C
D) All contain the same number of molecules.
Correct Answer:
Verified
Q102: If the pressure in a gas container
Q113: You have three cylinders containing O2 gas
Q119: At what temperature does argon have a
Q120: The ratio of the rate of effusion
Q121: Which of the following would have a
Q122: What is the volume of 30.0 g
Q125: What is the lowest layer of the
Q126: What is the Celsius temperature of 100.0
Q127: A gas bottle contains 0. 350 mol
Q129: How many grams of F2 gas are
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents