A fixed amount of gas at 25.0 °C occupies a volume of 20.0 L when the pressure is 0.8386 bar. Use Charles's law to calculate the volume (L) the gas will occupy when the temperature is increased to 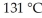 while maintaining the pressure at 0.8386 bar.
while maintaining the pressure at 0.8386 bar.
A) 14.8 L
B) 105 L
C) 3.82 L
D) 22.4 L
E) 27.1 L
Correct Answer:
Verified
Q101: A container filled with gas is connected
Q104: What is the pressure in a gas
Q110: The volume of 350.mL of gas at
Q129: How many grams of F2 gas are
Q130: A 45.0 L steel tank at 20.0
Q131: Give the formula for ozone.
A) O3
B) O2
C)
Q132: A 2.75 L container filled with CO2
Q133: This equation is used to calculate the
Q136: A 0. 286 g sample of gas
Q137: How many molecules of N2 are in
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents