Lithium and nitrogen react to produce lithium nitride: 6Li(s) + 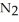 (g) →
(g) → 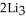 N(s)
N(s)
How many moles of lithium nitride are produced when 0.450 mol of lithium react in this fashion?
A) 0.150
B) 0.900
C) 0.0750
D) 1.35
E) 0.225
Correct Answer:
Verified
Q101: Which of the compounds H2C2O4,Ca(OH)2,KOH,and HI behave
Q102: In an acid-base neutralization reaction,38.74 mL of
Q119: Which one of the following compounds behaves
Q128: Which substance is the limiting reactant when
Q130: If the percent yield for the following
Q144: What is the oxidation number of the
Q152: What is the oxidation number of the
Q174: Magnesium burns in air with a dazzling
Q176: When 280. mL of 1.50 × 10-4
Q178: What is the molarity of a NaOH
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents