Automotive air bags inflate when sodium azide decomposes explosively to its constituent elements: 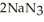 (s) → 2Na(s) +
(s) → 2Na(s) + 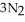 (g) How many moles of
(g) How many moles of 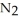 are produced by the decomposition of 2.54 mol of sodium azide?
are produced by the decomposition of 2.54 mol of sodium azide?
A) 1.69
B) 7.62
C) 3.81
D) 0.847
E) 1.27
Correct Answer:
Verified
Q7: What are the coefficients in front of
Q111: When dissolved in water,KOH behaves as:
A)an acid
Q149: What is the oxidation number of the
Q161: A solution is prepared by adding 1.40
Q162: Lithium and nitrogen react to produce lithium
Q165: What is the oxidation number change for
Q168: Automotive air bags inflate when sodium azide
Q169: How many millilitres of 0.152 mol L-1
Q170: A solution is prepared by mixing 70.0
Q171: Lithium and nitrogen react in a combination
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents