Calcium oxide reacts with water in a combination reaction to produce calcium hydroxide: CaO(s) + 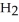 O(l) →
O(l) → 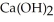 (s)
(s)
A 4.50 g sample of CaO is reacted with 4.34 g of 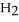 O. How many grams of water remain after the reaction is complete?
O. How many grams of water remain after the reaction is complete?
A) 0.161 g
B) 0.00893 g
C) 1.45 g
D) 0.241 g
E) 2.89 g
Correct Answer:
Verified
Q102: How many moles of CuO can be
Q121: How many grams of calcium chloride are
Q134: Sodium metal and water react to form
Q135: If the percent yield for the following
Q149: What is the concentration (M)of CH3OH in
Q152: What is the concentration (M)of a NaCl
Q157: Balance the chemical equation given below,and determine
Q201: Calculate the number of grams of solute
Q202: How many millilitres of a 9.0 mol
Q203: What is the concentration of HCl in
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents