How many millilitres of a stock solution of 13.1 mol L-1 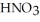 would be needed to prepare 0.500 L of 0.500 mol L-1
would be needed to prepare 0.500 L of 0.500 mol L-1 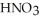 ?
?
A) 0.0191 mL
B) 52.4 mL
C) 0.0524 mL
D) 3.28 mL
E) 19.1 mL
Correct Answer:
Verified
Q136: What is the concentration of FeCl3 in
Q148: What is the concentration of NO3- ions
Q150: How many grams of CH3OH must
Q154: If the reaction of phosphate ion with
Q157: A student prepared a stock solution by
Q214: A stock solution of HNO3 is prepared
Q217: How many grams of H3PO4 are in
Q218: How many grams of NaOH (MW =
Q220: A FeCl3 solution is 0.175 mol L-1.
Q221: What is the molar concentration of sodium
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents