 Table 2-14
Table 2-14
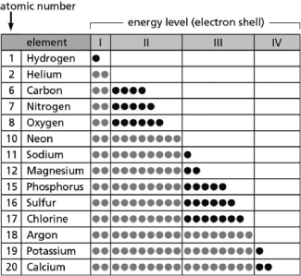
-Table 2-14 indicates the number and arrangement of electrons in the first four atomic electron shells for selected elements.On the basis of the information in the chart and what you know about atomic structure, which elements will form ions with a net charge of +2 in solution?
A) carbon; sulfur
B) helium; neon
C) sodium; potassium
D) magnesium; calcium
Correct Answer:
Verified
Q1: Select the answer that BEST completes the
Q2: If the isotope 32S has 16 protons
Q3: Avogadro's number was established as the total
Q4: There are 90 naturally occurring elements on
Q5: Carbon 14 is an unstable isotope of
Q7: Which subatomic particles contribute to the atomic
Q8: Which subatomic particles can vary between isotopes
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents