Table 2-14 indicates the number and arrangement of electrons in the first four atomic electron shells for selected elements.Use the information in the table to fill in the blanks for A-E.There may be more than one answer for each. 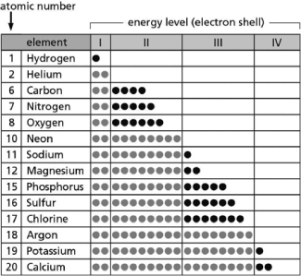
Table 2-14
A.__________ are chemically inert.
B.__________ form ions with a net charge of +1 in solution.
C.__________ form stable but reactive diatomic gases.
D.__________ form ions with a net charge of ?1 in solution.
E.__________ form ions with a net charge of +2 in solution.
Correct Answer:
Verified
B....
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q37: _ play an important role in organizing
Q38: Aromatic carbon compounds such as benzene are
Q39: Which of the following expressions accurately describes
Q40: Substances that release protons when they dissolve
Q41: Indicate whether the statements below are TRUE
Q43: Because there are four different monomer building
Q44: The variety and arrangement of chemical groups
Q45: Indicate whether the statements below are TRUE
Q46: There are 20100 different possible sequence combinations
Q47: A.If 0.5 mole of glucose weighs 90
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents