The anhydride formed between a carboxylic acid and a phosphate (Figure 3-45A) is a high-energy intermediate for some reactions in which ATP is the energy source.Arsenate can also be incorporated into a similar high-energy intermediate in place of the phosphate (Figure 3-45B) .Figure 3-45C shows the reaction profiles for the hydrolysis of these two high-energy intermediates.What is the effect of substituting arsenate for phosphate in this reaction? (A) 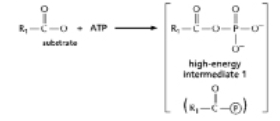
(B) 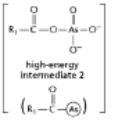
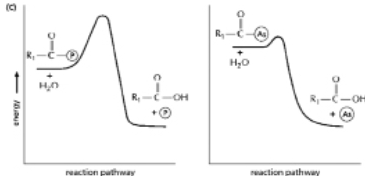
Figure 3-45
A) It forms a high-energy intermediate of lower energy.
B) It forms a high-energy intermediate of the same energy.
C) It decreases the stability of the high-energy intermediate.
D) It increases the stability of the high-energy intermediate.
Correct Answer:
Verified
Q53: Fill in the blanks, selecting from the
Q54: The synthesis of glutamine from glutamic acid
Q55: NADH and NADPH are activated carrier molecules
Q56: Indicate whether the following statements are TRUE
Q57: Indicate whether the following statements are true
Q59: Indicate whether the following statements about enzymes
Q60: Indicate whether the following statements are TRUE
Q61: In general, there is a positive change
Q62: Coenzyme A can be converted to acetyl
Q63: Although the biochemical study of reaction rates
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents