Using the table of bond dissociation energies, the OH for the following gas- phase reaction is
KJ) 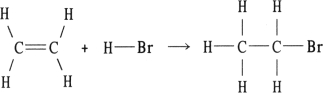

A) - 291
B) - 57
C) 2017
D) 291
E) - 356
Correct Answer:
Verified
Q76: A double bond consists of _ pairs
Q82: There are paired and unpaired electrons in
Q83: The ion NO- has _ valence electrons.
A)
Q84: The ability of an atom in a
Q85: The Lewis structure of PF3 shows that
Q87: Using the table of average bond energies
Q88: How many equivalent resonance forms can be
Q89: Using the table of average bond energies
Q90: The electron configuration of the phosph?ide ion
Q91: The only noble gas without eight valence
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents