Based on entropy considerations alone, which homogeneous aqueous equilibrium would be expected to lie to the right?
A) AgI2- + 2Br- 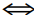 AgBr2- + 2I-
AgBr2- + 2I-
B) Fe(NH3) 62+ + C20H10N42- 11ec835b_aed0_b9fc_b7a5_cf026f9e50f9_TB34225555_11Fe(NH3) 2(C20H10N4) + 4NH3
C) CoCl42+ + 6H2O 11ec835b_aed0_b9fc_b7a5_cf026f9e50f9_TB34225555_11Co(H2O) 62+ + 4Cl-
D) Ni(H2NC2H4NH2) 32+ + 6NH3 11ec835b_aed0_b9fc_b7a5_cf026f9e50f9_TB34225555_11Ni(NH3) 62+ + 3H2NC2H4NH2
E) Cu(NH3) 42+ + 6H2O 11ec835b_aed0_b9fc_b7a5_cf026f9e50f9_TB34225555_11Cu( H2O) 62+ + 4NH3
Correct Answer:
Verified
Q13: Which one of the following coordination compounds
Q14: Which of the following can form both
Q15: Which of the following will display optical
Q16: How many bonds can ethylenediamine form to
Q17: Based on the crystal- field strengths F-
Q19: Linkage isomerism would most likely occur when
Q20: What are the donor atoms in a
Q21: Which complex below has 2 unpaired electrons?
A)
Q22: Complexes containing metals with d10 electron configurations
Q23: The chelate effect is best attributed to
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents