Which of the following equations represents the roasting of an ore?
A) All of these represent roasting processes.
B) PbO (s) + CO (g) 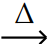 Pb (l) + CO2 (g)
Pb (l) + CO2 (g)
C) HgS (s) + O2 (g) 11ec835a_37ed_5a1a_b7a5_d3a2a63a892c_TB34225555_11Hg (g) + SO2 (g)
D) 2ZnS (s) + 3O2 (g) 11ec835a_37ed_5a1a_b7a5_d3a2a63a892c_TB34225555_112ZnO (s) + 2SO2 (g)
E) 2MoS2 (s) + 7O2 (g) 11ec835a_37ed_5a1a_b7a5_d3a2a63a892c_TB34225555_112MoO3 (s) + 4SO2 (g)
Correct Answer:
Verified
Q45: Which one of the following is false
Q46: A disadvantage to leaching gold with aqueous
Q47: The hydrated manganese(II) ion is
A) blue.
B) violet.
C)
Q48: The major impurities found in bauxite are
Q49: An alloy is a
A) a mineral containing
Q51: The Hall process is
A) the pyrometallurgic process
Q52: The molecular- orbital model for Ge shows
Q53: Which statement about steel is false?
A) It
Q54: Which mineral contains aluminum?
A) malachite
B) bauxite
C) cinnabar
D)
Q55: If the electronic structure of a solid
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents