Which of the following equations represents a calcination?
A) PbCO3 (s) 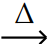 PbO (s) + CO2 (g)
PbO (s) + CO2 (g)
B) CaO (l) + SiO2 (l) 11ec835a_37ed_5a1a_b7a5_d3a2a63a892c_TB34225555_11CaSiO3 (l)
C) HgS (s) + O2 (g) 11ec835a_37ed_5a1a_b7a5_d3a2a63a892c_TB34225555_11Hg (g) + SO2 (g)
D) PbO (s) + CO (g) 11ec835a_37ed_5a1a_b7a5_d3a2a63a892c_TB34225555_11Pb (l) + CO2 (g)
E) All of the above are calcination processes.
Correct Answer:
Verified
Q42: A material that contains more than one
Q69: A slag is formed when a basic
Q70: Which one of the following is not
Q72: What is the purpose of adding zinc
Q73: Write the two reactions that are used
Q75: What is the name and the formula
Q77: In the Bayer process, the purpose of
Q79: An aqueous solution of Fe(NO3)3 will slowly
Q134: Most transition metal ions contain partially occupied
Q135: _ arises when the unpaired electrons of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents