What is the coefficient of NO2 when the following disproportionation reaction is balanced
__________ ? 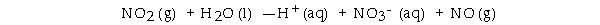
A) 4
B) 1
C) 3
D) 2
E) 5
Correct Answer:
Verified
Q62: In metallic hydrides,the oxidation number of hydrogen
Q153: The disilicate ion is .
A) Si2O86-
B)
Q155: The nitride ion is a strong BrØnsted-
Q156: Hydrogen can have oxidation states of .
A)
Q157: The dissolution of 1.0 mol of to
Q159: The correct name of H2CO3 is _
Q160: Pyrex glass is formed by adding an
Q184: Why are nitric acid solutions sometimes yellowish?
Q188: The instability of xenon fluorides is due
Q194: Oxides can react with water to form
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents