The missing product in this reaction combines with oxygen to form a compound with the formula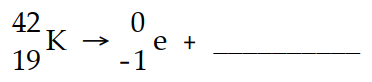
A) M2O3
B) M3O2
C) M2O
D) MO2
E) MO
Correct Answer:
Verified
Q105: On average, neutrons are produced by every
Q106: The nuclear disintegration series of is the
Q107: Due to the nature of the positron,
Q108: What percentage of electricity generated in the
Q111: How many neutrons are emitted when a
Q112: Radium undergoes alpha decay. The product of
Q114: The missing product in this reaction would
Q165: Positron emission causes a decrease of one
Q171: The neutron/proton ratio of stable nuclei increases
Q174: In the formula k = 0.693/t1/2,k is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents