The mass of a proton is 1.00728 amu and that of a neutron is 1.00867 amu. What is the mass defect (in amu) of a 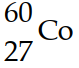 nucleus? (The mass of a cobalt- 60 nucleus is 59.9338 amu.) .?
nucleus? (The mass of a cobalt- 60 nucleus is 59.9338 amu.) .?
A) 0.0662
B) 0.5405
C) 27.7830
D) 0.4827
E) 0.5489
Correct Answer:
Verified
Q116: Who is credited with first achieving fission
Q117: Nuclei above the belt of stability can
Q118: 41Ca decays by electron capture. The product
Q119: In balancing the nuclear reaction 
Q146: The relative biological effectiveness (RBE)is 10 fold
Q169: In radioactive dating,the ratio of carbon-12 to
Q171: The neutron/proton ratio of stable nuclei increases
Q176: When an isotope undergoes electron capture,what happens
Q177: Gamma radiation only changes the atomic number
Q178: The SI unit of an absorbed dose
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents