The half- reaction occurring at the anode in the balanced reaction shown below is . 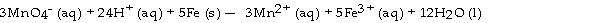
A) 2MnO4- (aq) + 12H+ (aq) + 6e- - 2Mn2+ (aq) + 3H2O (l)
B) Fe (s) -Fe2+ (aq) + 2e-
C) Fe (s) -Fe3+ (aq) + 3e-
D) Fe2+ (aq) - Fe3+ (aq) + e-
E) MnO4- (aq) + 8H+ (aq) + 5e- -Mn2+ (aq) + 4H2O (l)
Correct Answer:
Verified
Q38: Using Table 20.1, which substance can oxidize
Q39: The standard emf for the cell using
Q41: The more the value of E°red, the
Q42: How many minutes will it take to
Q44: What is the oxidation number of manganese
Q45: Corrosion of iron is retarded by .
A)
Q46: How many seconds are required to produce
Q47: The standard cell potential (E°cell) for the
Q48: The balanced half- reaction in which dichromate
Q106: The quantity of charge passing a point
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents