is the oxidizing agent in the reaction below. 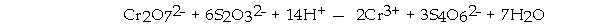
A) S4O62-
B) Cr2O72-
C) H+
D) Cr3+
E) S2O32-
Correct Answer:
Verified
Q51: The balanced half- reaction in which sulfate
Q52: A voltaic cell can be constructed of
Q53: 1V = .
A) 1 C/J
B) 1 J/s
C)
Q54: The standard cell potential (E°cell) for the
Q55: The standard cell potential (E°cell) of the
Q60: The standard cell potential (E°cell) for the
Q61: Which substance is serving as the reducing
Q85: At constant temperature and pressure the Gibbs
Q105: When iron is coated with a thin
Q109: The anode of the alkaline battery is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents