The element X has three naturally occurring isotopes. The isotopic masses (amu) and % abundances of the isotopes are given in the table below. The average atomic mass of the element is
Amu) 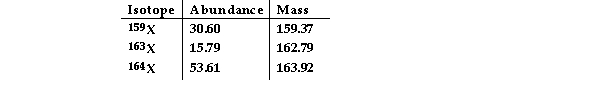
A) 162.35
B) 163.15
C) 161.75
D) 162.03
E) 33.33
Correct Answer:
Verified
Q47: Which isotope has 36 electrons in an
Q48: Which pair of elements would you expect
Q49: In the symbol below, X =
Q50: Which one of the following species has
Q51: Which metal does not require to have
Q53: An ion has 8 protons, 9 neutrons,
Q54: How many protons does the Br- ion
Q55: Of the three types of radioactivity characterized
Q56: Which pair of elements is most apt
Q57: A molecular formula always indicates .
A) the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents