Silver has two naturally occurring isotopes with the following isotopic masses: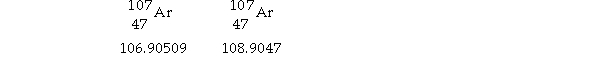
The average atomic mass of silver is 107.8682 amu. The fractional abundance of the lighter of the two isotopes is .
A) 0.7578
B) 0.9047
C) 0.5184
D) 0.2422
E) 0.4816
Correct Answer:
Verified
Q70: Gravitational forces act between objects in proportion
Q71: All atoms of a given element have
Q72: Which one of the following polyatomic ions
Q73: An atom of 17O contains protons.
A) 17
B)
Q74: The element X has three naturally occurring
Q76: Consider the following selected postulates of Dalton's
Q77: Which one of the following is not
Q78: Which atom has the largest number of
Q79: In the symbol shown below, x =
Q80: Which one of the following is the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents