The element X has two naturally occurring isotopes. The masses (amu) and % abundances of the isotopes are given in the table below. The average atomic mass of the element is amu. 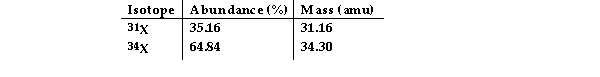
A) 34.02
B) 30.20
C) 35.22
D) 32.73
E) 33.19
Correct Answer:
Verified
Q108: Carbon can exist in different forms called
Q109: Oxygen forms an ion with a charge
Q110: Which species below is the sulfite ion?
A)
Q111: Which element in the halogen family would
Q112: Aluminum reacts with a certain nonmetallic element
Q114: The formula of a salt is XCl2.
Q115: The correct name for CaH2 is _
Q117: Oxygen is a _ and nitrogen is
Q118: Elements in Group 2A are known as
Q243: The formula for potassium sulfide is _.
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents