For the reaction 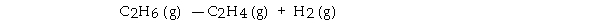 ΔH° is +137 kJ/mol and ΔS° is +120 J/K · mol. This reaction is .
ΔH° is +137 kJ/mol and ΔS° is +120 J/K · mol. This reaction is .
A) spontaneous only at high temperature
B) spontaneous at all temperatures
C) spontaneous only at low temperature
D) nonspontaneous at all temperatures
Correct Answer:
Verified
Q6: ΔS is negative for the reaction .
A)
Q7: The value of ΔG° at 100.0 °C
Q8: With thermodynamics, one cannot determine .
A) the
Q9: The entropy of the universe is .
A)
Q10: The thermodynamic quantity that expresses the degree
Q12: A common name for methanol (CH3OH) is
Q13: The standard Gibbs free energy of formation
Q14: A system that doesn't exchange matter or
Q15: Of the following, only _ is not
Q16: Consider the reaction:![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents