The value of ΔG° at 141.0 °C for the formation of phosphorous trichloride from its constituent elements, 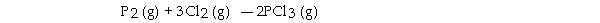 is kJ/mol. At 25.0 oC for this reaction, ΔH° is - 720.5 kJ/mol, ΔG° is - 642.9 kJ/mol, and
is kJ/mol. At 25.0 oC for this reaction, ΔH° is - 720.5 kJ/mol, ΔG° is - 642.9 kJ/mol, and
ΔS° is - 263.7 J/K.
A) - 611.3
B) - 829.7
C) 3.65 × 104
D) 1.08 × 105
E) - 683.3
Correct Answer:
Verified
Q25: Given the following table of thermodynamic data,
Q26: For an isothermal process, OS = .
A)
Q27: When a system is at equilibrium, _.
A)
Q28: For a reaction to be spontaneous under
Q29: Which one of the following correctly indicates
Q31: The first law of thermodynamics can be
Q32: OS is positive for the reaction _.
A)
Q33: The value of ΔG° at 100.0 °C
Q34: Of the following, the entropy of gaseous
Q35: Which one of the following processes produces
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents