Classify the following compounds as weak acids (W) or strong acids (S) :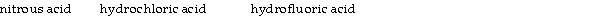
A) S S S
B) W W W
C) S W W
D) W S W
E) W S S
Correct Answer:
Verified
Q15: A 0.1 M solution of has a
Q16: The magnitude of Kw indicates that .
A)
Q17: Calculate the molarity of hydroxide ion in
Q18: The molar concentration of hydronium ion in
Q19: A BrØnsted- Lowry acid is defined as
Q21: Which one of the following is the
Q22: Using the data in the table, which
Q23: Of the acids in the table below,
Q24: An aqueous solution at 25.0 °C contains
Q25: The hydride ion, H- , is a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents