The equilibrium constant for the gas phase reaction 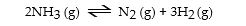
Is Keq = 230 at 300 °C. At equilibrium, _ _.
A) products predominate
B) only reactants are present
C) reactants predominate
D) only products are present
E) roughly equal amounts of products and reactants are present
Correct Answer:
Verified
Q6: At 900 K, the equilibrium constant (Kp)
Q7: The Keq for the equilibrium below is
Q8: The equilibrium constant for reaction 1 is
Q9: The Keq for the equilibrium below is
Q10: The equilibrium constant (Kp) for the reaction
Q12: The relationship between the rate constants for
Q13: The Keq for the equilibrium below is
Q14: In what year was Fritz Haber awarded
Q15: Consider the following reaction at equilibrium:
Q16: Consider the following equilibrium.![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents