In which of the following reactions would increasing pressure at constant temperature not change the concentrations of reactants and products, based on Le Cha^telier's principle?
A) 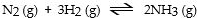
B) 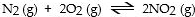
C) 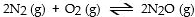
D) 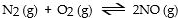
E) 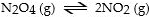
Correct Answer:
Verified
Q23: At 400 K, the equilibrium constant for
Q24: The Kp for the reaction below is
Q27: Dinitrogentetraoxide partially decomposes according to the following
Q29: At equilibrium, .
A)all chemical reactions have ceased
B)the
Q30: Which one of the following is true
Q31: The equilibrium constant for the gas phase
Q32: Consider the following reaction at equilibrium:
2NH3 (g)
Q33: Which one of the following will change
Q84: Pure _ and pure _ are excluded
Q95: The number obtained by substituting starting reactant
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents