The data in the table below were obtained for the reaction:
2 ClO2 (aq) + 2 OH- (aq) →ClO3- (aq) + ClO2- (aq) + H2O (1) 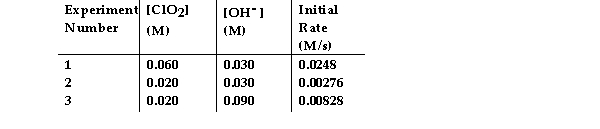
-What is the magnitude of the rate constant for the reaction?
A) 4.6
B) 115
C) 230
D) 713
E) 1.15 × 104
Correct Answer:
Verified
Q34: At elevated temperatures, methylisonitrile (CH3NC) isomerizes to
Q35: The reaction A (aq) - B (aq)
Q36: The half- life of a first- order
Q37: The isomerization of methylisonitrile to acetonitrile CH3NC
Q38: A particular first- order reaction has a
Q40: One difference between first- and second- order
Q41: The active site of nitrogenase is a
Q42: Nitrogen dioxide decomposes to nitric oxide and
Q110: The relationship of absorbed light to the
Q113: For the reaction aA + Bb →
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents