Which one of the following substances will not have hydrogen bonding as one of its intermolecular forces?
A) 
B) 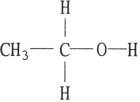
C) 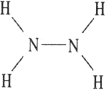
D) 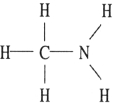
E) 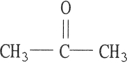
Correct Answer:
Verified
Q9: The phase diagram of a substance is
Q10: Based on the figure above, the boiling
Q11: Which statement is true about liquids but
Q12: A substance that expands to fill its
Q13: What is the predominant intermolecular force in
Q15: Which one of the following exhibits dipole-
Q16: What portion of the volume of each
Q17: Hydrogen bonding is a special case of
Q18: A gas is _ and assumes of
Q19: The vapor pressure of a liquid _.
A)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents