Which one of the following substances will have hydrogen bonding as one of its intermolecular forces?
A) 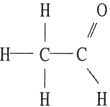
B) 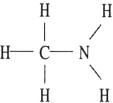
C) 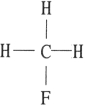
D) 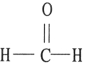
E) 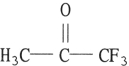
Correct Answer:
Verified
Q65: The phase diagram of a substance is
Q72: The phase diagram of a substance is
Q75: On a phase diagram, the critical temperature
Q76: On the phase diagram below, segment corresponds
Q77: The predominant intermolecular force in (CH3)2NH is
Q79: The scattering of light waves upon passing
Q81: Of the following substances, only has London
Q82: solids consist of atoms or molecules held
Q101: In general,intramolecular forces determine the _ properties
Q111: London Dispersion Forces tend to _ in
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents