Zinc reacts with aqueous sulfuric acid to form hydrogen gas: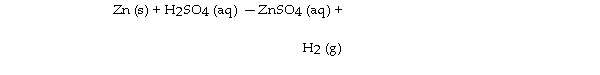
In an experiment, 201 mL of wet H2 is collected over water at 27 °C and a barometric pressure of 733 torr. The vapor pressure of water at 27 °C is 26.74 torr. The partial pressure of hydrogen in this experiment is _ atm.
A) 0.964
B) 706
C) 760
D) 0.929
E) 1.00
Correct Answer:
Verified
Q11: At STP, the ratio of the root-
Q12: "Isothermal" means .
A) that OHrxn = 0
B)
Q13: Of the following, has a slight odor
Q14: Of the following, is a valid statement
Q15: A fixed amount of gas at 25.0
Q17: In ideal gas equation calculations, expressing pressure
Q18: An ideal gas differs from a real
Q19: A real gas will behave most like
Q20: A 1.44- g sample of an unknown
Q21: Of the following gases, has density of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents