For the hypothetical reaction aA → products, the experimental data showed the following behavior (below) . What is the reaction order with respect to reactant A? 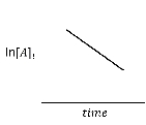
A) zero order
B) first order
C) second order
D) third order
E) fourth order
Correct Answer:
Verified
Q19: Which of the following expressions does
Q19: For a certain reaction of the general
Q20: Which of the given relationships correctly
Q22: A first-order reaction is 40.0% complete at
Q29: The rate constant of a first-order decomposition
Q30: The reaction A → B follows first-order
Q32: In a first-order reaction,the half-life is 133
Q37: For the second-order reaction below,the initial concentration
Q38: A second-order reaction starts with an initial
Q80: A first-order chemical reaction is observed to
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents