What is a possible set of quantum numbers for an unpaired electron in the orbital box diagram below? 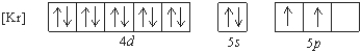
A) n = 1, = 1, = -1, ms = +1/2
B) n = 4, = 2, = -1, ms = -1/2
C) n = 5, = 2, = -2, ms = +1/2
D) n = 5, = 0, = 0, ms = -1/2
E) n = 5, = 1, = -1, ms = +1/2
Correct Answer:
Verified
Q21: All of the following ground-state electron configurations
Q23: If the ground state electron configuration of
Q29: For which of the following atoms is
Q31: Which of the following orbital diagrams represents
Q36: What noble gas core precedes the valence
Q36: Hund's rule states that the most
Q37: Which of the following elements has the
Q37: Which is the correct valence shell
Q38: Which element has the following ground state
Q39: What is the ground-state electron configuration of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents