The mass spectrum of an element with two naturally occurring isotopes is shown below. What is the best estimate of the element's (average) atomic weight? 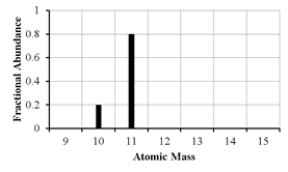
A) 10 amu
B) 11 amu
C) 10.8 amu
D) 10.2 amu
E) 10.5 amu
Correct Answer:
Verified
Q25: What is the common name of the
Q27: Naturally occurring element X exists in three
Q28: Which of the following statements is not
Q31: Which of the following elements is present
Q33: Which of the following elements belongs to
Q34: Which of the following statements is
Q34: Lithium has two naturally occurring isotopes,6Li and
Q40: Rubidium has two naturally occurring isotopes.The atomic
Q41: What is the charge on the sodium
Q53: Which of the following sets of ions
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents