Which of the following reactions would provide the highest yield of 3-bromohexane?
A) 
B) 
C) 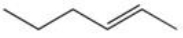
D) 
E) All would provide equal yields of 3-bromohexane.
Correct Answer:
Verified
Q26: What is the structure of the
Q27: The compound ortho-dichlorobenzene has substituents on which
Q28: When 4,4-dimethyl-2-pentene adds H2 in an addition
Q29: What is the structure and name of
Q30: How many moles of hydrogen gas (H2)are
Q32: Which of the following terms best describes
Q33: What is the geometry and bond angle
Q34: What is the molecular formula of the
Q35: What is the IUPAC name of the
Q36: Which of the following is the correct
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents