Two molecules of ethane experience what type of attractive forces? 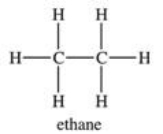
A) London dispersion forces
B) dipole-dipole interactions
C) hydrogen bonding
D) ionic bonding
E) covalent bonding
Correct Answer:
Verified
Q1: Which of the following statements correctly describes
Q2: Which statement INCORRECTLY describes what happens to
Q3: Consider 1.00 L of air in a
Q5: What is the volume occupied by a
Q6: Which of the following represent STP (Standard
Q7: Which of the following is(are)NOT a type
Q8: Which statement concerning the three states of
Q9: What law predicts the expansion of a
Q10: At 100°C,which gas sample exerts the greatest
Q11: Which of the following is NOT a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents