What is wrong with the Lewis structure shown for sulfur trioxide,SO3? 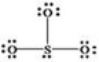
A) Oxygen,not sulfur,should be the central atom.
B) Sulfur should have 10 electrons around it instead of 8,to show its expanded octet.
C) The structure shows 26 valence electrons,but there should only be 24.
D) There are too many bonding electrons shown.
E) The structure should show each oxygen atom with a double bond to the sulfur atom.
Correct Answer:
Verified
Q18: What is the three-dimensional arrangement of positive
Q19: What do the dots in a Lewis
Q20: What kind of bonding exists in substances
Q21: Which properly represents the Lewis structure
Q22: What is the formula of the ionic
Q24: The drug "lithium" is often prescribed to
Q25: Baking soda consists of the ionic compound
Q26: Vehicle airbags inflate when the ionic compound
Q27: Predict the formula of the compound formed
Q28: Formaldehyde is a covalent compound used as
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents