How many bonding electrons are present in the Lewis structure for the bicarbonate ion,shown below? 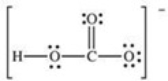
A) 4
B) 5
C) 8
D) 10
E) 24
Correct Answer:
Verified
Q37: What is the name of CuF2 in
Q38: The ionic compound iron(II)sulfate is used in
Q39: How many dots are present in the
Q40: Which statement about general bonding characteristics is
Q41: In the compound shown below,how can the
Q43: In the molecule AX2,the central atom A
Q44: If the shape of a molecule is
Q45: Which element has the greatest electronegativity?
A)Si
B)P
C)Cl
D)Ar
E)Br
Q46: What is the Stock name of Cu+?
A)cupric
Q47: The ionic compound magnesium hydroxide can be
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents