An electron is in the n = 3 energy level in a hydrogen atom. To ionize this atom, it is necessary for the electron to gain a minimum of how much energy? See Figure 4-11. 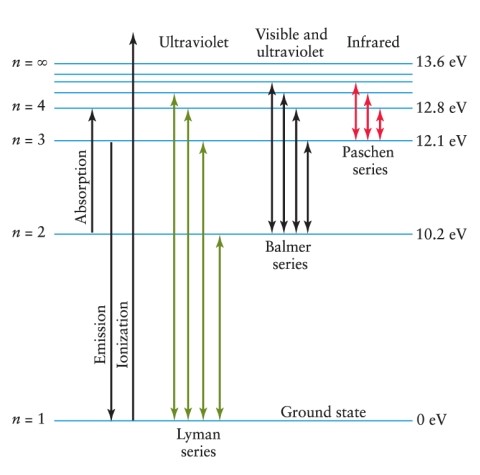
A) 1.5 eV
B) 4.5 eV
C) 12.1 eV
D) 13.6 eV
Correct Answer:
Verified
Q183: The spectrum of a star shows a
Q184: An object is observed to show no
Q185: The observed change in the wavelength of
Q186: A source emitting waves of constant wavelength
Q187: Hydrogen gas emits a strong spectral
Q189: A police radar device bounces radio
Q190: An astronomer observing the spectrum of
Q191: When electromagnetic radiation (e.g., light) is Doppler-shifted
Q192: The orbits of electrons in an atom
Q193: An electron is in the n =
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents