The monosaccharide derivative shown below bonds to hydrophobic molecules in the liver.What effect does this have on the molecule to which it binds? 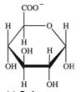
A) It increases the hydrophobicity of the molecule, making it more soluble in fatty tissues.
B) It increases the polarity of the molecule, making it more soluble in bodily fluids, and more readily removed.
C) It eliminates the ability of the molecule to act as a nutritional source of energy.
D) It decreases the polarity of the hydrophobic molecule, allowing it to be retained in the body longer.
E) It increases the nutritional value of the molecule by allowing it to be retained by the body longer.
Correct Answer:
Verified
Q8: What two monosaccharides combine to form sucrose?
A)glucose
Q22: The Fischer projection for the monosaccharide ribulose
Q22: What term describes a carbon atom that
Q23: What is the simplest type of carbohydrate?
A)sugars
B)monosaccharides
C)cellulose
D)starch
E)None
Q27: What term properly describes the relationship between
Q27: Disaccharides and polysaccharides contain monosaccharide units joined
Q29: What is the molecular formula of glucose?
A)C6H12O5
B)C6H12O6
C)C6H10O5
D)C5H10O5
E)C9H18O9
Q32: What two functional groups are present in
Q33: What type of compound is produced in
Q38: What complex carbohydrate provides a source of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents