Lidocaine is used as a local anesthetic and a cardiac depressant.When Lidocaine is administered, it is administered as the alkylammonium salt Lidocaine HCl.Why is the alkylammonium salt, rather than the free amine, used? 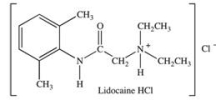
A) The alkylammonium salt has a higher solubility in water and bodily fluids than the free amine.
B) The alkylammonium salt is not as addictive as the free amine.
C) The alkylammonium salt is more basic than the free amine.
D) The alkylammonium salt is a gas at room temperature and can be readily inhaled.
E) None of these are correct.
Correct Answer:
Verified
Q62: Purine and pyrimidine rings are found in
Q64: Which statement concerning amines and amides is
Q66: Amides may be prepared by which of
Q67: N-methyl-1-propanamine contains a methyl group,hydrogen,and a propyl
Q70: Amines behave as bases when dissolved in
Q71: A primary amide has two carbons bonded
Q76: What is a medical use for barbiturates?
A)analgesic
B)anticonvulsant
C)sedative
D)stimulant
E)Both
Q80: Hydrolysis of an amine produces an amide.
Q81: Serotonin is a neurotransmitter.
Q82: Proteins are polymers of amino acids joined
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents