When a hemiacetal reacts with an alcohol, an acetal is formed.This is how disaccharides and polysaccharides are formed.What is the structure of the acetal formed in the following reaction? 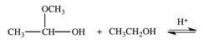
A) 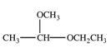
B) 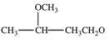
C) 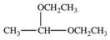
D) 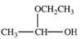
E) 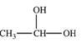
Correct Answer:
Verified
Q42: Small (low molecular weight)aldehyde molecules are generally
Q43: The most oxidized organic form of a
Q45: A ketone can be reduced to a
Q49: Which of the following pairs of reactant
Q50: The compound shown below is the enol
Q54: Pure formaldehyde is a liquid at room
Q55: Formaldehyde is used to kill viruses without
Q57: A ketone will react with Tollens' reagent
Q59: The oxidation of isopropyl alcohol produces propanone.
Q63: In any pair of keto-enol tautomers,the keto
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents