Which statement best describes the solubility of the alcohol whose structure is shown below? 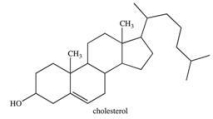
A) It would be readily soluble in water because it contains a polar hydroxyl group.
B) It would be very soluble in water because it can hydrogen bond with water.
C) It would have limited solubility in water because the molecule is largely hydrophobic.
D) It would be soluble in NaCl(aq) because it can dissociate into ions.
E) It is impossible to predict solubility from the structure alone.
Correct Answer:
Verified
Q33: Which type of alcohol does NOT undergo
Q34: What is the IUPAC name of the
Q35: What is the structure of the ether,
Q39: What is the common name of the
Q39: Zaitsev's rule can be used to predict
Q40: Which of the following structures represents the
Q41: What type of compound is produced by
Q41: Which statement best explains why alcohols have
Q47: What is the name of the product
Q50: When an alcohol is heated with concentrated
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents