Ammonia is synthesized industrially by the reaction of nitrogen and hydrogen in the presence of an iron catalyst according to the reaction equation below.What effect would removing the iron catalyst have on this reaction? 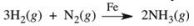
A) The reaction would shift to the right, producing more ammonia.
B) The reaction rate would increase.
C) The reaction would shift to the left, and consume ammonia.
D) The reaction rate would decrease.
E) There would be no effect on the reaction since Fe is not consumed in the reaction.
Correct Answer:
Verified
Q5: The rate of a chemical reaction increases
Q8: What instrument is used for measuring the
Q13: A carbohydrate sample weighing 0.235 g was
Q14: Which statement concerning a reversible reaction at
Q14: Which of the following is FALSE about
Q18: Which statement concerning energy changes in chemical
Q19: How many small calories are equivalent to
Q21: Under normal conditions,which of the following would
Q38: A reversible reaction is said to have
Q50: A reaction will be spontaneous at all
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents