The reaction shown below is at equilibrium.Use LeChatelier's principle to predict the effect of adding ammonia gas to the equilibrium reaction mixture. 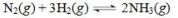
A) The equilibrium position will remain unchanged.
B) The equilibrium position will shift to the right.
C) The equilibrium position will shift to the left.
D) The equilibrium constant will increase.
E) All of the nitrogen gas will be used up.
Correct Answer:
Verified
Q26: In kinetics,the order of a reaction
A)is the
Q27: Which term describes the measure of the
Q28: A granola bar contains 185 nutritional Calories.How
Q28: Which one of the following,if changed,would change
Q29: One effect of a catalyst being added
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents