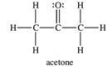
When acetone (a component of nail polish remover) evaporates, hydrogen bonds between acetone molecules must be broken up.
Correct Answer:
Verified
Q61: Metals conduct electricity well due to the
Q65: The density of hydrogen gas is lower
Q66: Ionic compounds tend to have higher melting
Q67: Detergents and soaps decrease the surface tension
Q68: The attractive forces between acetone molecules are
Q68: Polar gases exhibit more ideal behavior than
Q69: Dalton's Law states that the volume of
Q71: As temperature increases,viscosity decreases.
Q71: Ethylene glycol molecules have a greater attraction
Q76: The boiling point of a liquid is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents