Formaldehyde is a covalent compound used as a preservative for biological specimens.Which statement concerning the Lewis structure of formaldehyde, shown below, is FALSE? 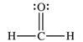
A) Four electrons are being shared between the carbon and oxygen atoms.
B) The oxygen atom has four valence electrons that are not being shared.
C) The oxygen and carbon atoms each have an octet of electrons in their valence shells.
D) The hydrogen atoms have incomplete valence shells.
E) There are a total of 12 valence electrons in formaldehyde.
Correct Answer:
Verified
Q1: What is the name of the ionic
Q4: What does it mean if an atom
Q10: A molecule of deoxyribose,an essential part of
Q15: Which combination of atoms is most likely
Q20: What is the formula of the ionic
Q22: Vehicle airbags inflate when the ionic compound
Q31: What is the name of the ion
Q33: What statement about the ammonia molecule,NH3,is FALSE?
A)The
Q34: At what temperature is a liquid converted
Q40: Which statement about general bonding characteristics is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents