Which of the following Lewis structures represent resonance forms of ozone, O3? 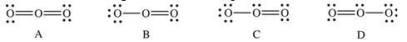
A) A and B
B) B and C
C) C and D
D) A and C
E) A and D
Correct Answer:
Verified
Q27: Predict the formula of the compound formed
Q29: What is the formula of the sulfate
Q35: What is wrong with the Lewis structure
Q35: What is the name of the compound
Q36: Baking soda consists of the ionic compound
Q40: What is the Lewis structure of methanethiol,
Q44: In the compound shown below, how can
Q45: Which element has the greatest electronegativity?
A)Si
B)P
C)Cl
D)Ar
E)Br
Q55: What is the name of Fe2(SO4)3 in
Q77: How many total valence electrons are in
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents