According to the VSEPR theory, what is the geometry (shape) around the phosphorus atom in the Lewis structure shown below? 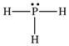
A) linear
B) bent (angular)
C) trigonal planar
D) trigonal pyramidal
E) tetrahedral
Correct Answer:
Verified
Q62: Which of the following bonds would be
Q63: A covalent bond is formed when
A)two metals
Q69: Which of the following would NOT describe
Q70: There are three atoms of iodine represented
Q73: What is the formula of iron(III) bromide?
A)Fe(Br2)3
B)Fe3Br
C)Fe3Br3
D)FeBr3
E)Fe2Br3
Q78: Which of the following is an essential
Q79: Which compound contains a central atom with
Q81: As a rule,ionic compounds tend to have
Q85: Ionic solids are amorphous.
Q92: Six electrons shared between two atoms corresponds
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents